Oxidative stress monitoring analysis aimed at evaluating the genotoxic potential of free radicals
GENOXPACE
Did you know? The air you breathe, the stress you feel, and daily habits could be silently harming your DNA and your long-term wellbeing.
GENOXPACE measures the level of the biomarker 8-OHdG (8-hydroxy-2’-deoxyguanosine), a quantitative and qualitative indicator used to monitor the accumulation of genotoxicity and the associated risk of developing degenerative and cancer-related diseases.
This biomarker provides an overall measure of the genotoxic burden accumulated over time, particularly in cases of chronic exposure to oxidative stressors such as pollutants, radiation, or inflammation.
ORIGIN AND IMPACT OF GENOTOXICITY
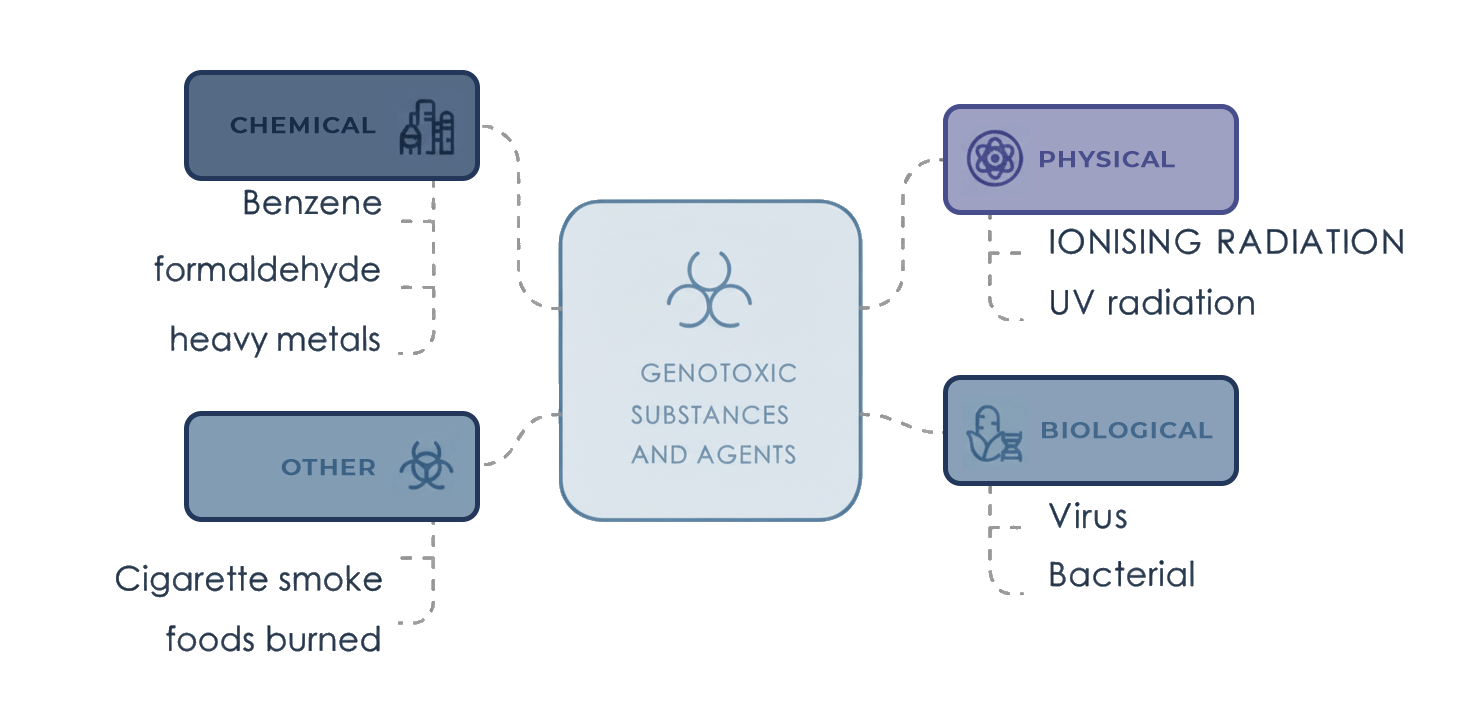
Genotoxicity refers to the ability of certain chemical, physical or biological agents to damage the DNA within cells, causing somatic mutations that contribute to genomic instability and increase the risk of disease development.
Measuring the accumulation of genotoxic damage represents an advanced approach to preventing ageing and the onset of age-related diseases.
When DNA is damaged by a genotoxic substance, the cell initiates repair mechanisms. However, this repair process is not always accurate, and may result in alterations to the DNA sequence—thus producing a somatic mutation.
Somatic mutations may have a variety of consequences depending on the specific gene affected:
- Some mutations may be silent or have no apparent effect,
- Others may lead to significant health conditions, such as cancer.
Genotoxicity is therefore recognised as one of the primary causes of somatic mutations. The progressive accumulation of such mutations over time plays a central role in biological ageing and in the development of age-related diseases.
Examples of known genotoxic substances and/or agents:
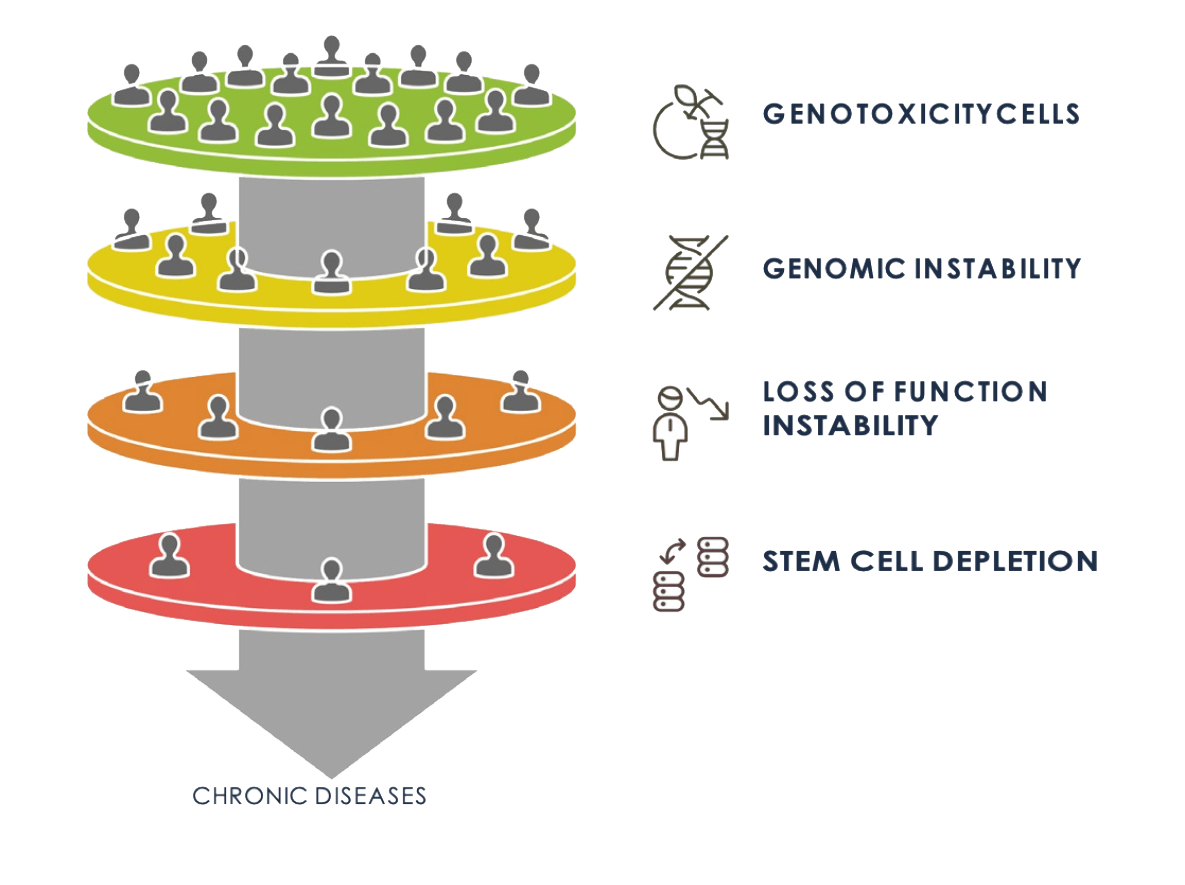
DNA damage resulting from genotoxic exposure may lead to a wide array of health consequences, including:
Genetic Mutations
Mutations in DNA sequences may lead to inherited genetic disorders or the development of cancer
Cellular Damages
Damage can impair the function of stem cells, thereby diminishing the regenerative capacity of tissues and organs and accelerating the ageing process.
Stem Cell Depletion
Genotoxicity may reduce the number and functionality of stem cells in various tissues, thereby compromising tissue repair mechanisms.
Chronic Diseases
There is an increased risk of developing chronic illnesses such as cancer, cardiovascular diseases, and neurodegenerative conditions.
Hereditary Diseases
If genotoxic damage occurs in germ cells (sperm or ova), it may be transmitted to offspring, leading to inherited disorders.
Accelerated Ageing
Damaged cells may work less efficiently or die prematurely, leading to visible and systemic signs of ageing like wrinkles, hair loss, fatigue, and cognitive decline.
Reproductive Issues
Genotoxic exposure may impact fertility and increase the risk of miscarriage or congenital abnormalities.
Immune Dysfunction
Genotoxic agents can compromise the immune system’s ability to respond to infections and disease, increasing vulnerability to pathogens.
Neurological Issues
These may include memory impairment, concentration difficulties, mood changes, and an elevated risk of neurodegenerative disorders.
Cardiovascular Risks
Exposure to genotoxic substances may raise the risk of heart disease, stroke, and hypertension.
Respiratory Problems
Such as asthma, chronic bronchitis, and, in rare cases, lung cancer.
Digestive Disturbances
Symptoms may include nausea, vomiting, diarrhoea, and abdominal discomfort.
Impact on Stem Cells
Stem cells, which are essential for tissue regeneration and maintenance, are particularly vulnerable to oxidative damage and genomic instability. Mutations and alterations in their DNA can lead to:
- Loss of functionality – Damaged stem cells lose their ability to self-renew and to differentiate correctly.
- Depletion of the stem cell pool – Cumulative damage can reduce the reservoir of regenerative cells, impairing the body’s ability to repair tissues.
- Development of chronic diseases – The accumulation of genetic errors may promote the onset of degenerative, autoimmune, or cancerous diseases.
Oxidative Damage and Systemic Inflammation
Low-grade chronic inflammation, also known as systemic inflammation, is closely associated with oxidative damage.
This type of inflammation is characterized by a persistent but mild activation of the immune system, often triggered by factors such as obesity, stress, chronic infections, or exposure to environmental toxins.
Chronic oxidative damage initiates a dysfunctional inflammatory response, creating a vicious cycle: oxidative damage fuels inflammation, and inflammation generates further free radicals. This interaction between oxidative damage and low-grade chronic inflammation is one of the leading contributors to long-term health deterioration.
The level of systemic inflammation can be easily detected through the CYTOXPACE test.
Measuring the accumulation of genotoxicity in the human body offers several advantages in the prevention of ageing and age-related diseases, including:
ADVANTAGES OF GENOTOXICITY MEASUREMENT
Comprehensive Analysis
| BIOMARKER | REFERENCE VALUE |
| 8-OHdG concentrazione (media) | 0,94 – 60 ng/dl |
| OPTICAL DENSITY | 0.0 – 2.5 nm |
Why GENOXPACE
Discover all the advantages of measuring the level of the biomarker 8-OHdG
| Prevention and management of cancer risk |
| Monitoring in cardiovascular diseases |
| Assessment of risk for neurodegenerative diseases |
| Monitoring in metabolic diseases |
| Evaluation in environmental exposure contexts |
| Monitoring of ageing |
| Oxidative DNA damage (such as 8-OHdG) is associated with the risk of genomic mutations and tumor development. |
| Oxidative stress contributes to endothelial dysfunction and the progression of cardiovascular conditions (hypertension, atherosclerosis, myocardial infarction). |
| Oxidative stress and DNA damage are implicated in the pathogenesis of
neurodegenerative diseases (Alzheimer’s, Parkinson’s, ALS). |
| 8-OHdG is a useful biomarker for tracking oxidative damage associated with chronic hyperglycemia and diabetic
complications (nephropathy, retinopathy). |
| Exposure to environmental or occupational factors may increase oxidative stress and DNA damage (cigarette smoke, air pollutants, heavy metals, radiation). |
| Ageing is associated with increased oxidative stress and a decline in DNA repair capacity. |
| To identify potential DNA damage and assess oncological risk. |
| To monitor oxidative stress and prevent cardiovascular diseases. |
| To assess DNA damage in patients with or at risk of neurodegenerative conditions. |
| To monitor oxidative damage in individuals with diabetes or other metabolic diseases. |
| To assess the impact of such exposures and the associated long- term DNA damage risk. |
| To monitor ageing-related changes and support strategies to slow them down. |
EXTENDED DIAGNOSTIC PROTOCOL
As part of an integrated approach, it is recommended to complement the analysis of systemic inflammation with the following tests, which form the BIOXPACE protocol :
SENEXPACE
SENEXPACE – analyses cellular senescence, a natural defence mechanism that prevents the uncontrolled proliferation of cells with damaged DNA and, consequently, high tumourigenic potential. Senescent cells release inflammatory signals that generate and spread inflammation throughout the body, thereby triggering systemic inflammation.
CYTOXPACE
CYTOXPACE – assesses key markers of systemic inflammation that, when altered, are associated with accelerated aging processes, increased genotoxicity, and genomic instability, conditions that increase the risk of disease onset.
IMMUNEXPACE
IMMUNEXPACE – evaluates the balance and effectiveness of the immune system in carrying out its functions. The immune system helps counteract systemic inflammation by eliminating senescent cells that fuel inflammatory processes. When the immune system is weakened, these cells are not adequately removed and therefore accumulate in tissues, contributing to chronic inflammation.
GUTXPACE
GUTXPACE – analyses the gut microbiome to identify and characterize the microorganisms present in the intestinal tract. The balance of intestinal bacteria is one of the main modulators of immune responses. Gut health directly influences the effectiveness of the immune system, including its ability to clear senescent cells that drive systemic inflammation.(LINK)
NANOXPACE
NANOXPACE – detects and quantifies nano- and microplastics in the bloodstream. The immune system recognizes these foreign substances, triggering an inflammatory response. The progressive accumulation of nano/microplastics contributes to genotoxicity and therefore genomic instability, which in turn accelerates degenerative processes and ageing.
Ready to Take the Next Step?
Your journey to better health starts with a simple conversation
Our team of medical experts and dedicated patient coordinators is here to guide you every step of the way. From your initial consultation to post-treatment follow-up, we provide personalized support tailored to your unique needs. Schedule a free consultation today and discover how our advanced treatments can help you achieve your health goals.
