Understanding your microbiome means understanding your health
MICROBALANCE
Scientific analysis of the intestinal and vaginal microbiome to detect imbalances and support personalized preventive strategies.
Bioscience Institute’s MICROBALANCE test provides a comprehensive platform for analyzing the intestinal and vaginal microbiota through advanced Next Generation Sequencing (NGS) technology.
By identifying microbial imbalances and guiding personalized interventions, MICROBALANCE supports prevention strategies and promotes long-term well-being through science-based insights.
What is the Microbiota?
The human body is not a sterile environment — it hosts an extraordinary ecosystem of microorganisms (bacteria, fungi, protozoa, and viruses) that coexist with our cells and play a key role in maintaining health. Together, these microbes form the microbiota, while the total collection of their genetic material is known as the microbiome.
Intestinal and Vaginal Microbiota
The microbiota colonizes multiple areas of the body — from the skin to the digestive, respiratory, and urogenital tracts.
In particular:
- The intestinal microbiota (often called the “gut flora”) contains around 100 trillion bacteria, primarily located in the colon. Its genome — the gut microbiome — includes up to 100 times more genes than the human genome, influencing digestion, immunity, and metabolism.
- The vaginal microbiota, by contrast, is a more specialized ecosystem, whose balance protects against infections and supports reproductive health.
Why Monitor the Microbiota?
The microbiota acts as a biological shield, protecting the body against pathogens that may colonize the skin, mouth, gut, or reproductive tract.
Its balance directly affects immunity, metabolism, and overall physiological function.
When this balance is disrupted — a condition known as dysbiosis — harmful microbes can proliferate, contributing not only to digestive disorders, but also to systemic issues such as inflammaging (age-related chronic inflammation).
Monitoring the microbiota helps:
- evaluate the effectiveness of dietary or pharmacological treatments,
- guide personalized nutrition,
- prevent recurrent infections, and
- maintain optimal health through targeted, evidence-based interventions.
Clinical Indications
MICROBALANCE analysis is particularly recommended for individuals who:
- suffer from intestinal disorders (colitis, diarrhea, constipation, flatulence, or irregularity);
- experience recurrent genitourinary infections (cystitis, vaginitis, candidiasis, urethritis);
- are in pregnancy or breastfeeding, to ensure adequate maternal and neonatal microbiota;
- are in menopause, to support metabolic and physiological adaptation;
- present risk factors for intestinal or systemic diseases;
- are following nutritional or pharmacological treatments aimed at restoring balance;
- wish to implement preventive lifestyle strategies at any age.
Microbiota and Health Conditions
Each individual’s microbiota is unique, and certain microbial profiles are associated with better health outcomes.
Through genomic sequencing, MICROBALANCE provides valuable insights to support prevention and wellness strategies across several domains, including:
- Immune system regulation, inflammation, and inflammaging
- Genitourinary infections and vaginal dysbiosis
- Obesity and metabolic syndrome
- Oncology, in relation to microbiome-related tumor risk
- Pregnancy and breastfeeding health
- Mood and behavior, via the gut–brain axis
| Pathology | Microbiota alteration |
|---|---|
| intestinal inflammation | Enterobacteriaceae (increase), Clostridia (decrease) |
| Crohn’s disease | Escherichia coli, Yersinia, Clostridium difficile |
| rheumatoid arthritis | Prevotellaceae |
| constipation | Enterobacteriaceae, methanogens (increase) |
| irritable bowel syndrome | Ruminococcaceae (decrease) |
| cholelithiasis | Mollicutes (decrease) |
| urinary incontinence | Odoribacteraceae (decrease) |
| acne | Deltaproteobacteria (decrease) |
| osteoarthritis | Lentisphaeria (decrease) |
| food allergies | Comamonadaceae, Enterococcaceae, Bacteroidaceae (increase); Bifidobacteriaceae, Ruminococcaceae, et al. (decrease) |
| obesity | Firmicutes/Bacteroidetes ratio increase |
| type 2 diabetes | Escherichia coli (increase), Clostridia (decrease) |
| stomach cancer | Helicobacter pylori |
| colorectal cancer | Prevotella (increase) |
Cytokines and Health
Pro-inflammatory cytokines regulate immune and inflammatory responses.
When their production is temporary, they support healing and defense.
When sustained, they contribute to tissue damage and disease progression.
High cytokine and CRP levels are correlated with a greater risk of cardiovascular disease and cancer, including colorectal and breast cancer.
Monitoring these markers enables early preventive action and supports personalized longevity strategies.
How the Test is Performed
The MICROBALANCE test analyzes the genetic material of the microorganisms that live in the intestine and vagina through Next Generation Sequencing (NGS), a state-of-the-art technology for identifying bacterial composition and balance within the microbiota.
Sample collection is simple and non-invasive: it can be comfortably performed at home using the sampling kit provided by the Bioscience Institute, which includes all the necessary materials and detailed instructions.
Once collected, the sample is shipped to the Institute’s laboratories using the dedicated transport system included in the kit to ensure proper preservation.
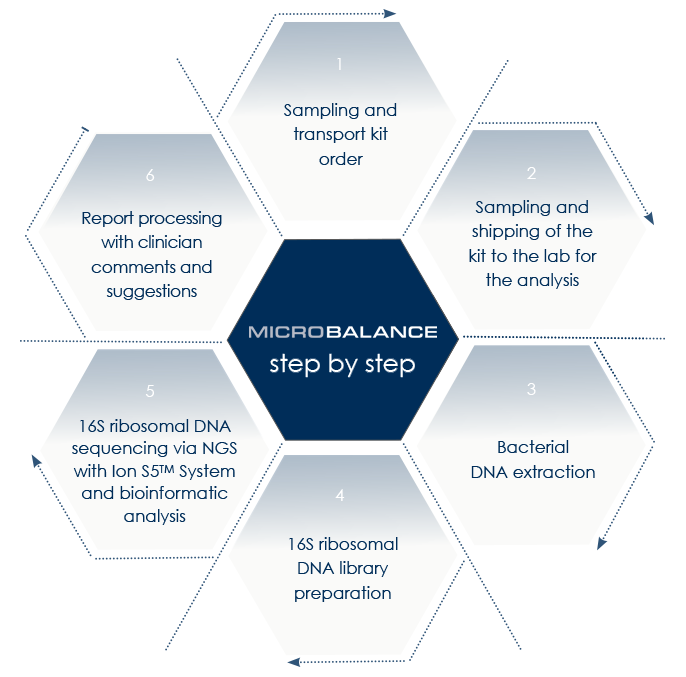
The laboratory workflow follows a validated six-step analytical process:
- Sampling and transport kit order
- Sampling and shipment to the laboratory
- Bacterial DNA extraction
- 16S ribosomal DNA library preparation
- NGS sequencing and bioinformatic analysis
- Report generation and clinical interpretation
The resulting microbiota profile does not represent a medical diagnosis, but rather a scientific evaluation of microbial composition and balance.
Interpreted by qualified physicians and nutritionists, the report supports the development of personalized nutritional strategies and the assessment of potential benefits from targeted food supplements, aimed at restoring or maintaining microbiota equilibrium.
In-depth Information
Would you like to explore further or learn more? Access comprehensive explanations by expanding the sections below.
MICROBIOME, AGING AND INFLAMMAGING
Aging not only alters organs and tissues but also the microbiota, that is the community of microbes that live in the organism and on its surface. Some changes in this microbial population are lifestyle-dependent; others are instead a consequence of the passing of the years. Acting on them can help you age while staying healthy.
The microbiome analysis makes it possible to identify any alterations of the microbiota by studying its genes and consequently, to develop the most suitable strategies to promote a good health by correcting the imbalances detected.
Aging and alterations of the intestinal microbiota
During aging, the intestinal microbiota undergoes several changes, such as:
- its ability to ferment carbohydrates decreases;
- it can ferment proteins better;
- its diversity is reduced, and some species of microbes increase relative to others.
Examples of gut microbiota bacteria that can change during aging include:
- Proteobacteria, associated with inflammation (local and systemic)
- Akkermansia muciniphila, responsible for the degradation of mucin (one of the components of the intestinal barrier)
- Bifidobacteria, anaerobic bacteria, which exert a range of beneficial effects on health
- Escherichia coli, a potentially pathogenic bacterium
- Microbes producing butyrate, a molecule that participates in the regulation of inflammation.
Some of the changes detectable with microbiome analysis are fundamental characteristics of the microbiota with advancing age and cannot be significantly changed by external actions. On others it is possible to act by playing on lifestyle, for example on nutrition and the intake of drugs that affect the state of the intestinal flora.
Intestinal dysbiosis in aging
Intestinal dysbiosis (changes in the intestinal microbiota) that occur during aging can be caused by:
- a diet with little variety
- an increase in sugar consumption
- an increase in fat consumption
- a reduction in the consumption of food of plant origin
- a reduction in the consumption of healthy foods
- an unhealthy lifestyle
In turn, intestinal dysbiosis is associated with:
- nervous system degeneration associated with the intestine
- alterations in intestinal motility
- reduction of the intestinal barrier
- increased intestinal permeability
Intestinal microbiota and inflammaging
Alterations in the intestinal microbiota also participate in the development and maintenance of so-called inflammaging, the low-grade inflammatory condition associated with aging. In fact, the compromise of the intestinal barrier and permeability can lead to the passage into the blood of molecules deriving from the microbiota. These molecules can create an inflammatory condition by activating a particular class of white blood cells (macrophages) and thus increase the risk of atherosclerosis. In turn, atherosclerosis is associated with cardiovascular disease and vascular dementia.
Furthermore, intestinal dysbiosis associated with aging can endanger cognitive abilities by causing changes in molecules associated with inflammation (cytokines) and fatty acids (called “short-chain fatty acids”). Finally, they can be associated with the release of a toxin (lipopolysaccharide, LPS) that promotes the formation of the beta-amyloid protein responsible for Alzheimer’s disease.
When to perform the microbiome analysis
The microbiome analysis with the Bioscience Institute MICROBALANCE during aging is particularly recommended in the case of:
- intestinal problems (colitis, diarrhea, constipation, flatulence, intestinal irregularity)
- genitourinary infections (cystitis, urethritis, candidiasis)
- menopause
- risk factors for intestinal or systemic diseases
- unbalanced diet
MICROBIOME AND GENITO-URINARY INFECTIONS
Vaginal and urinary tract infections are often recurring problems. The use of antibiotics and other specific drugs is not always useful against relapses, and the infection can return with an almost periodic rhythm. The solution can be hidden among the microbes that live right in the genito-urinary tract.
The vagina and urinary tract are not sterile environments. Analyzing the genome of the microbes that populate them (the microbiome) can help fight recurring infections.
The microbiota and dysbiosis of the genito-urinary tract
Numerous microbes live on the surface and in the human organism, which together form the so-called microbiota. The genito-urinary tracts are also populated by microorganisms; in conditions of equilibrium this coexistence does not constitute a danger, indeed, some microbes are precious allies of health. Lactobacilli, for example, help protect the vaginal environment from colonization by potential pathogens.
Sometimes the microbiota can undergo risky changes. These conditions, known as dysbiosis, are characterized by the reduction of health-friendly microbes and the increase of potential pathogens. Bacterial vaginosis, for example, is associated with the reduction of lactobacilli and the increase in Streptococcus, Staphylococcus or Enterobacteriaceae species, Candida, or Trichomonas. Recurrent urinary tract infections, on the other hand, are associated with a reduction in hydrogen peroxide-producing lactobacilli and with significant increases in various strains of Escherichia coli, often carriers of multiple drug resistance.
Intestinal and vaginal microbiota and genitourinary infections
The microbes that cause genito-urinary tract infections can come from the gut microbiota, that is, from the population of microorganisms living in the gut. From here the microbes can pass into the vagina, causing bacterial vaginosis for example.
From the vagina, microbes can then move on to colonize the area around the urethra, through which they can go up to the bladder and, sometimes, to the kidneys, causing urinary tract infections. The intestinal microbiota can for example be the source of Escherichia coli.
At other times, it is the vaginal microbiota that is a reservoir of pathogens independent of the intestinal microbiota. Not only that, the loss of lactobacilli species with protective functions that normally live in the vagina increases both the risk of bacterial vaginosis and infections by pathogens such as Neisseria gonorrhoeae, and that of urinary tract infections.
Factors that can alter the vaginal microbiota include exposure to antimicrobials and the use of some spermicides.
When to analyze the microbiome
Targeted approaches to fight the alteration of the intestinal and vaginal microbiota can help reduce the risk of urinary tract infections and disorders associated with the vaginal microbiota. Dysbiosis can, for example, be corrected with adequate nutrition, or with the intake of probiotic, prebiotic or symbiotic supplements..
The microbiome analysis with the MICROBALANCE test by Bioscience Institute makes it possible to evaluate the involvement of intestinal and vaginal microbes in genitourinary infections, in the case of:
- bacterial vaginosis vaginal infections caused by changes in the balance of the vaginal microbiota
- vaginitis and vulvovaginitis inflammation or vagina and vulva inflammation which may be associated with bacterial vaginosis, candidiasis, Trichomonas infections, hormonal changes (such as those associated with pregnancy or menopause) or sensitivity to products such as vaginal sprays, douches, spermicides, soaps, cleansers or laundry softeners
- recurrent urinary tract infections kidney, urethra, bladder, or ureter infections that keep recurring, such as cystitis
MICROBIOME, OVERWEIGHT AND OBESITY
Overweight and obesity affect 42.5% of the Italian adult population. Excess weight is not the only characteristic of these conditions: both go hand in hand with changes in the population of microbes that live in the gut (the gut microbiota). Not only that, alterations in the intestinal microbiota (the so-called dysbiosis) are also associated with an increased risk of complications typical of obesity, such as diabetes.
The microbiome analysis makes it possible to identify dysbiosis and to implement strategies aimed at correcting those changes that increase the risks to health due to excess weight.
Microbiota and excess weight
The link between intestinal microbiota and obesity depends in part on the role played by the bacterial flora in regulating the energies obtained from food.
In fact, the bacteria that live in the intestine are responsible for the fermentation of food components (dietary fibers) that otherwise would remain undigested. This fermentation increases the energy that is obtained from a meal, increasing it by 2 Kcal for each gram of fiber.
The fermentation of fibers is not a negative phenomenon; on the contrary, a diet rich in fiber promotes the growth of a health-friendly microbiota. However, dysbiosis associated with excess weight can lead obese people to absorb more energy than slender people. Furthermore, the fermentation of fibers leads to the production of short-chain fatty acids, which inhibit the degradation of fats while stimulating their accumulation, that of triglycerides and the production of adipocytes (the cells of adipose tissue).
Microbiota and complications of obesity
Alterations in the gut microbiota also affect the risk of health problems closely associated with obesity.
- The reduction of clostridia and the increase in lactobacilli are associated with insulin resistance, the antechamber of diabetes.
- The decrease in the richness of the microbiota is associated with both insulin resistance and an alteration in the fat and cholesterol levels in the blood.
- The communication between the intestine and the brain and the signals directed towards the adipose tissue are also altered by intestinal dysbiosis.
- The changes in the gut flora typically associated with obesity alter the production of hormones and other molecules by the cells of the gut.
Characteristics of the microbiota associated with obesity
The main intestinal dysbiosis associated with obesity is the increased ratio of Firmicutes to Bacteroidetes. The other variations include:
- reduction of microbiota diversity
- increase in microbiota richness
- increase in Blautia hydrogenotrophica, Coprococcus catus, Eubacterium ventriosum, Ruminococcus bromii, Ruminococcus obeum, Lactobacillus reuteri
- reduction inBacteroides faecichinchillae, Bacteroides thetaiotaomicron, Blautia wexlerae, Clostridium boltae, Flavonifractor plautii, Methanobrevibacter smithii and several Bifidobacterium, Lactobacillus
MICROBIOME AND TUMORS
Pollution, nutrition, and bad habits are not the only external factors that can influence the development of tumors. The population of microbes living in the human body (the so-called microbiota) also plays a role in the development of cancer. As a result of microbiome analysis (MICROBALANCE) it is possible to identify changes in the microbiota associated with tumors and to correct them to reduce risk factors.
Microbiota, inflammation, and the immune system
The link between the microbiota and tumor development is complex. It can involve hormonal mechanisms and nerve pathways, or it can depend on the entry of bacteria, molecules of bacterial origin or toxins in the organism. The link between microbiota, inflammation and the immune system also plays a role. In particular, the factors that can play a role in the development of tumors include:
- innate immunity and acquired immunity
- the regulation of inflammation and oxidative stress
The role of bacteria
Sometimes tumor development depends on individual bacterial species. Some can produce toxins that affect inflammation levels, others can affect genome stability, and still others can regulate gene expression. For example, Helicobacter pylori (the gastric ulcer bacterium) is directly involved in the development of esophageal and stomach cancers.
Nutrition can also play its part. Some bacteria, for example, can metabolize red meat derivatives to produce molecules that can damage DNA. At other times, it is not specific bacteria that promote tumor development, but variations in the composition or density of the microbiota that come into play.
Bacteria against cancer
There is also no shortage of bacteria capable of exerting protective effects against cancer. Not only that, microbiota can also make an important contribution in determining the effectiveness of anticancer therapies and their side effects. Ruminococcaceae, Faecalibacterium and Bacteroides, for example, influence the response to immunotherapy.
Some prebiotics (substances that increase the growth or activity of the gut microbiota) can also help prevent cancer by acting as antioxidants and reducing inflammation.
MICROBIOME, PREGNANCY AND LACTATION
It is never too early to take care of the population of “friendly” microbes that live in our organism: the link between health and these microorganisms (known as “microbiota”) begins in the very early stages of life. Therefore, analyzing their genome (the so-called “microbiome”) can already protect your health during gestation. The microbiome analysis during pregnancy and breastfeeding can help ensure proper bacterial flora not only for the mother but also for her baby, protecting them from the development of infections and diseases.
The microbiota during pregnancy
INTESTINAL MICROBIOTA DURING PREGNANCY
Pregnancy is characterized by endocrine, metabolic and immune variations associated with significant changes in the gut microbiota. Some of these variations are entirely physiological and healthy; their appearance is due to the natural weight gain of the pregnant woman and the need to feed the developing fetus. Other changes could be associated with pregnancy complications or could impair the development of the baby’s gut microbiota.
VAGINAL MICROBIOTA DURING PREGNANCY
However, the gut microbiota isn’t the only one that changes during pregnancy. The vaginal microbiota – important because it helps fight infections – becomes more stable, but its diversity decreases. Lactobacilli species increase and the pH falls. Any loss of lactobacilli, which have protective functions, can increase the risk of both vaginal and urinary tract infections. Distinguishing physiological changes from potentially pathological ones is important because:
- the gut microbiota appears to play a role in intrauterine infections.
- the intestines of vaginally-born babies are colonized by bacteria present in the mother’s vagina and intestines. It has been hypothesized that in the event of a caesarean section, the absence of this transmission may have long-term consequences on the development of the immune system and on the child’s health, who would be more exposed to the risk of obesity, asthma and celiac disease.
Microbiota and breastfeeding
Breastfeeding is also essential for the development of the baby’s gut microbiota.
During the first year of life, the microbial composition of breast milk changes. Since the microbes present in breast milk come from the gut, it is also crucial to take care of the maternal gut microbiota during breastfeeding.
Why analyze the microbiome during pregnancy?
During pregnancy and breastfeeding, the microbiome analysis with the MICROBALANCE test of the Bioscience Institute helps to identify alterations in the maternal bacterial flora at risk of complications for the mother’s or child’s health. The collection of the material necessary for the microbiome analysis during pregnancy and lactation is a simple procedure that can be done using the kit provided by the Bioscience Institute.
MICROBIOME, MOOD AND BEHAVIOR
The mind, as we know, can influence the functioning of the digestive system. From the feeling of “butterflies in the stomach” to the intestinal manifestations of states of stress, emotions can trigger the most diverse gastrointestinal symptoms. But this association is two-way, and what happens in the gut can also affect mood and behavior. The condition of the population of microbes living in the intestine (the microbiota) is reflected in the brain with moods, changes in behavior and responsiveness to stress.
Studying the intestinal bacterial flora with a microbiome analysis can unmask and help correct any alterations to promote not only physical but also psychological well-being.
The gut-brain axis
The complex network of relationships that connects the intestine to the brain is called the “gut-brain axis“. The numerous nerve endings in which the intestine is rich come into play and for this characteristic it is often also called the “second brain“. The nerve fibers associated with the gut make up one of the components of this network, the enteric nervous system. The others are:
- the central nervous system (brain and spinal cord)
- the autonomic nervous system
- the hypothalamic-pituitary-adrenal axis (the circuit that coordinates the stress response systems)
The brain influences intestinal activity both through mechanisms depending on nerve cells and through hormonal mechanisms. On the one hand, the microbiota is another target of these mechanisms, while on the other hand it acts on the same intestinal cells influenced by brain activity. This way it contributes to the bidirectional interaction between the gut and the brain.
Microbiota and the brain
The mechanisms by which the microbiota sends signals to the brain involve neurotransmitters, hormones, and the immune system:
- In the intestine, it interacts with intestinal cells and with the enteric nervous system.
- It interacts directly with the central nervous system through nervous, hormonal, and metabolic pathways.
- It generates immune signals directed to the brain.
Alterations of the intestinal bacterial flora (dysbiosis) modify the inflammatory responses promoting a state of chronic inflammation. The latter may in turn be associated with mood and behavioral changes, increased responsiveness to stress and a higher incidence of associated disorders.
The microbiome analysis
With the microbiome analysis (the microbiota genome) it is possible to detect dysbiosis that could affect not only the state of physical health but also psychological health. MICROBALANCE by the Bioscience Institute allows you to conduct the necessary sampling in a simple way, comfortably at home, using the kit that is sent to your address.
Ready to Take the Next Step?
Your path to better long-term health starts with understanding what’s happening inside your body.
Our specialists and scientific advisors are here to guide you from test selection to result interpretation, offering clear insights into your microbiome balance, chronic inflammation markers, genetic predispositions, and more. We provide personalized support tailored to your wellness goals.
Book your free consultation today and discover how our advanced molecular tests can help you take control of your health with confidence and clarity.
