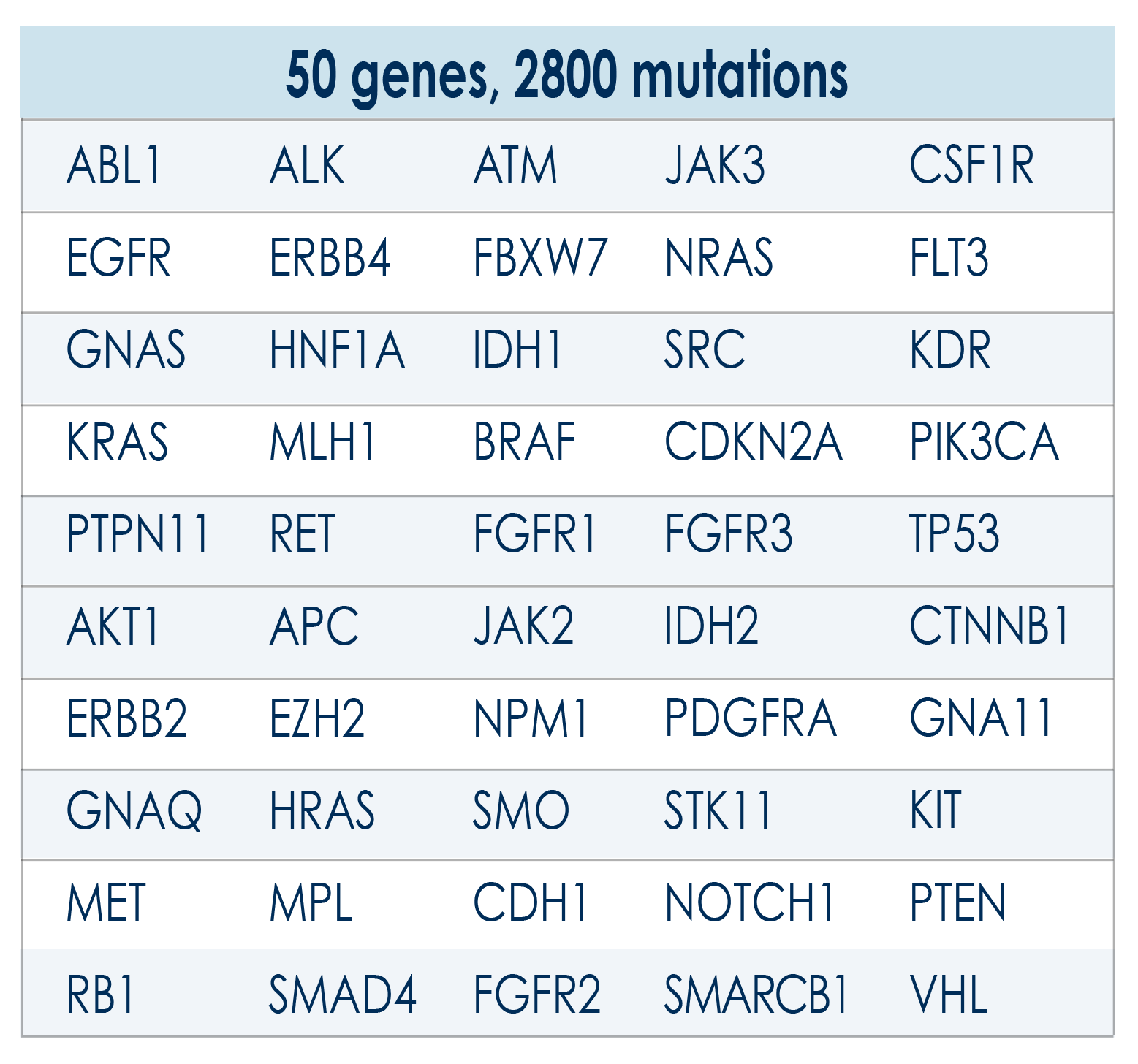
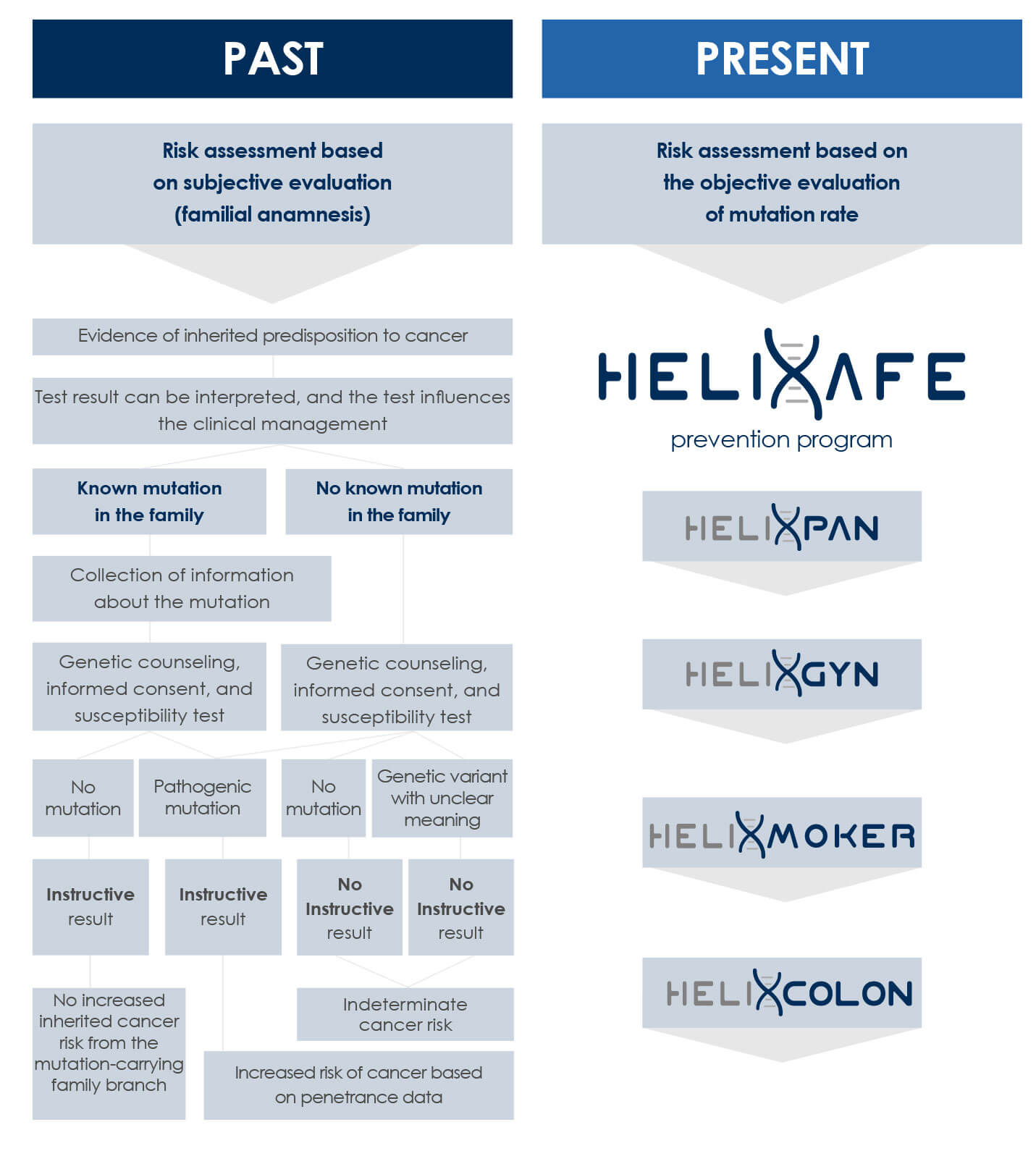
HELIXPAN is a prevention program aimed at evaluating the prodromal phase of solid tumors, except for those of the brain. With the annual repetition of the HELIXPAN test, the monitoring of somatic mutations is carried out and, therefore, genomic stability is kept under control which, when it is lacking, may lead to the onset of cancer

Solid Tumor Interception
Indications
The HELIXPAN test is suitable for anyone who does not live in conditions of environmental pollution, leads healthy lifestyles and has no reason to access targeted prevention programs.
An innovative model
With the Helixafe program, it is possible to evaluate objective parameters, such as somatic or acquired mutations, which can be analyzed using the most modern techniques of liquid biopsy and sequencing (Next Generation Sequencing). Thanks to NGS, it is possible to monitor the mutation frequencies in the patient and then analyze the genomic instability through the algorithm patented by the Bioscience Institute.
How the test is performed
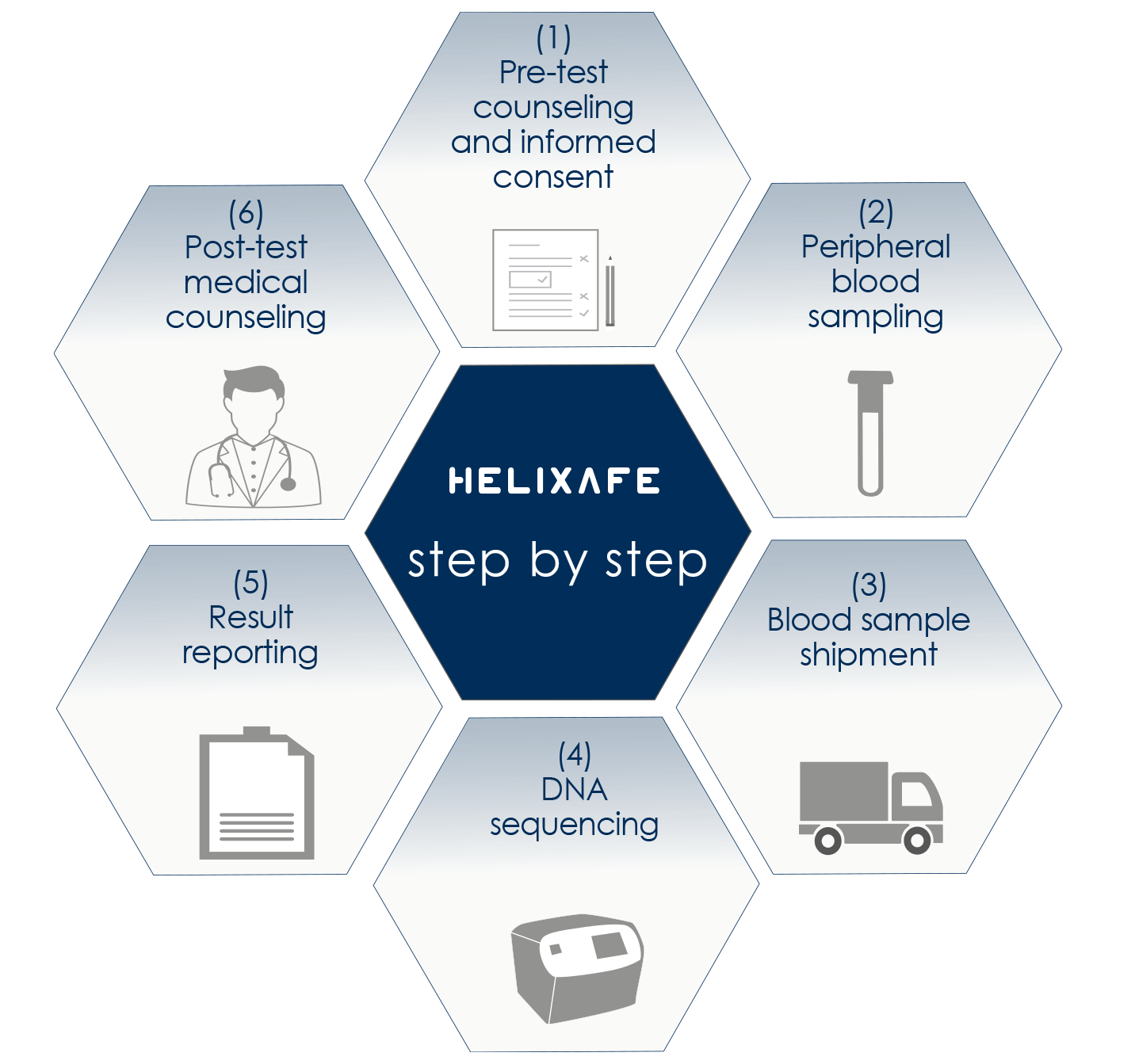
Bioscience Institute offers pre-test advice and provides all useful information about the HELIXAFE prevention program and the execution of the HELIXPAN test.
The test involves a simple blood sample that can be performed at one of our reference centers or through your doctor.
The Bioscience Institute laboratories extract the DNA present in the sample and sequence it using the advanced Next Generation Sequencing (NGS) techniques. The subsequent bioinformatics analysis allows the determination of the presence of any mutations in the genes under examination.
Test results are available in approximately 30 days.
Request HELIXPAN
Request the HELIXPAN test to know your level of genomic stability.
Email info@bioinst.com or fill out the form below to be contacted by one of our experts
(*) Required fields